Clinical Trials
Completed Trials:
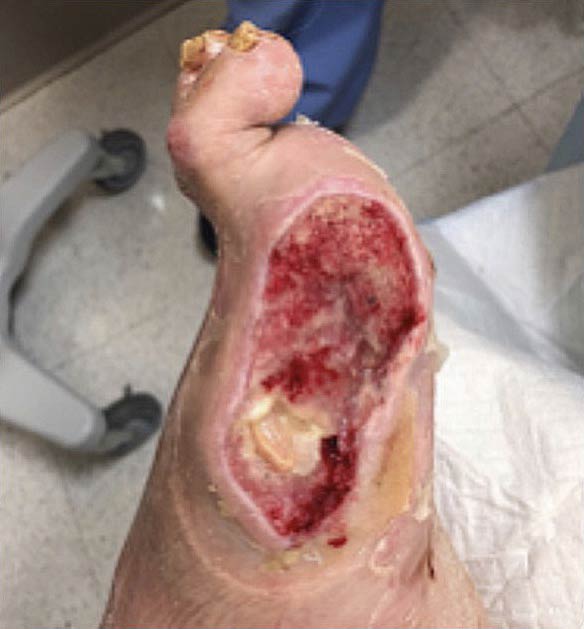
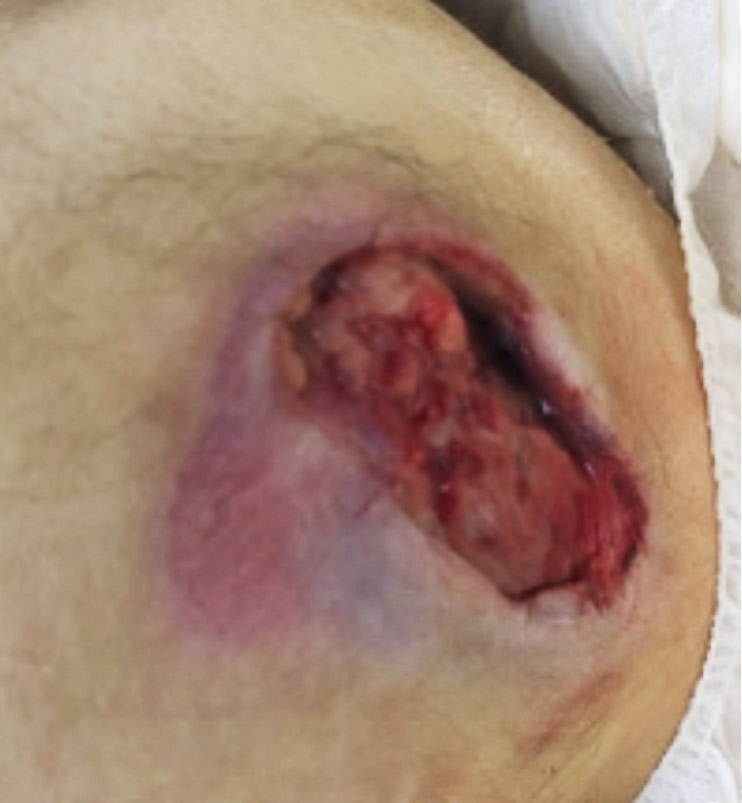
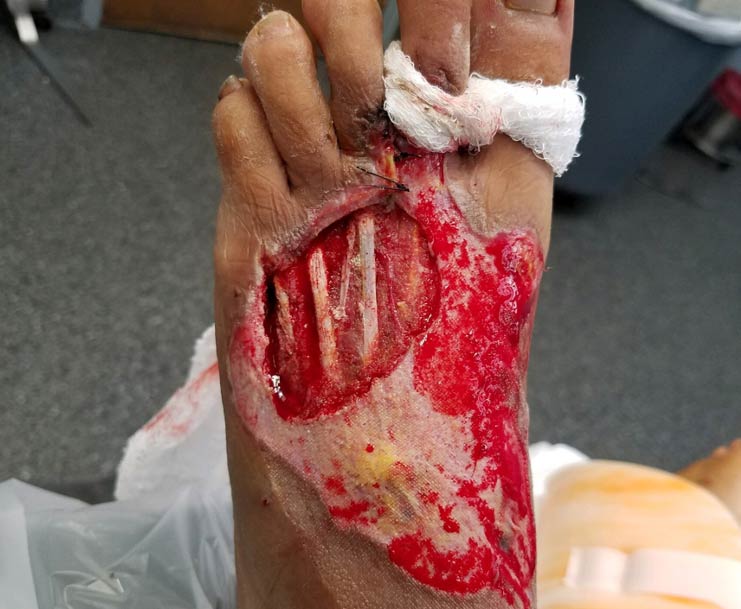
Diabetic Foot Ulcer (DFU) RCT
A Multi-center, Randomized Controlled Clinical Trial Evaluating the Effects of SkinTE® in the Treatment of Wagner One Diabetic Foot Ulcers — ClinicalTrials.gov Identifier: NCT03881254.
The trial met the primary endpoint of wound closure at 12 weeks with closure rates of 70% (35/50) of participants receiving SkinTE plus SOC versus 34% (17/50) of participants receiving SOC alone (p=0.00032). The secondary endpoint of Percent Area Reduction (PAR) assessed at 4, 6, 8, 10, and 12 weeks was also met with significantly greater PAR for the SkinTE plus SOC treatment group vs SOC alone (p=0.009). 90% (45/50) of SkinTE plus SOC treated participants received a single application of SkinTE. Treatment with SkinTE plus SOC increased the odds of wound closure by 5.37 times versus SOC alone (p=0.001).
Interim analysis was published in the International Wound Journal “A multicenter, randomized controlled clinical trial evaluating the effects of a novel autologous, heterogeneous skin construct in the treatment of Wagner one diabetic foot ulcers: Interim analysis”
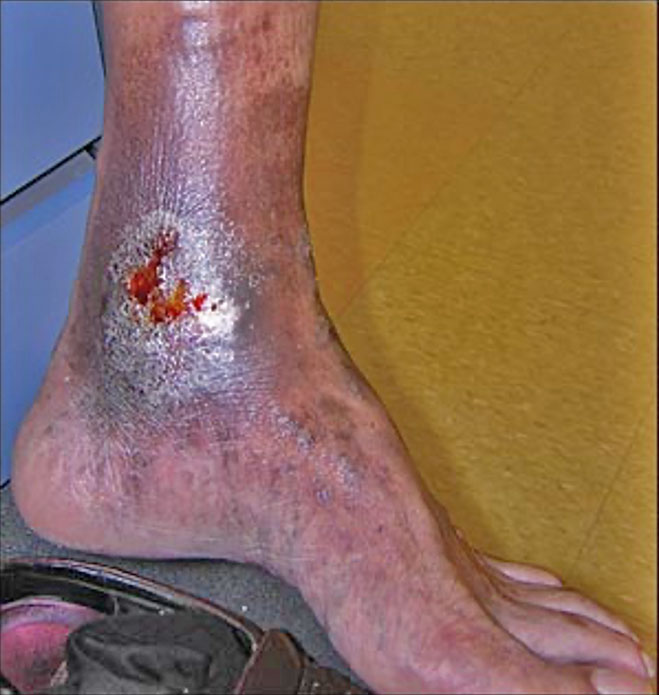
Venous Leg Ulcer (VLU) RCT:
A Multi-center, Randomized Controlled Clinical Trial Evaluating the Effects of SkinTE® in the Treatment of Venous Leg Ulcers—ClinicalTrials.gov Identifier: NCT03881267. The trial met the primary endpoint of wound closure at 12 weeks with closure rates of 71% (10/14) of participants receiving SkinTE plus SOC had wound closure at 12 weeks versus 33% (5/15) of participants receiving SOC alone (p=0.046). The secondary endpoint of Percent Area Reduction (PAR) assessed at 4, 6, 8, 10, and 12 weeks was also met with significantly greater PAR for the SkinTE plus SOC treatment group vs SOC alone (p=0.000035). 93% (13/14) of SkinTE plus SOC treated participants received a single application of SkinTE.
DFU Pilot Study:
SkinTE was used to treat 10 patients (11 DFUs) in a pilot trial. Results of the trial include: 10 of 11 (90.9%) DFUs healed within eight weeks of a single application of SkinTE; median time to closure was 25 days; DFU sizes ranged from 1.0 to 21.7 cm²; no SkinTE-related adverse reactions were observed.
Complete Wound Closure Following a Single Topical Application of a Novel Autologous Homologous Skin Construct: First Evaluation in an Open-Label, Single-Arm Feasibility Study in Diabetic Foot Ulcers. Int Wound J 2020 Oct;17(5):1366-1375.
VLU Pilot Study:
SkinTE was used to treat 10 patients in a pilot trial. Results of the trial include: 8 of 10 (80%) VLUs closed within 12 weeks of a single application of SkinTE; median time to closure was 21 days; and no SkinTE-related adverse reactions were observed.
Open-label Venous Leg Ulcer Pilot Study Using Novel Autologous Homologous Skin Construct. Plast Reconstr Surg Glob Open 2020 Jul 16;8(7):e2972.
Ongoing/Enrolling Trials:
COVER DFUs Trial:
Closure Obtained with Vascularized Epithelial Regeneration for DFUs With SkinTE®—ClinicalTrials.gov Identifier: NCT05372809